
Cynthia A. Janak
The truth about the HPV vaccines or what they do not want you to know (Part 3)
By Cynthia A. Janak
For those of you who have come here for the first time let me inform you as to why I am writing this series. I was part of an international group of women and we had a "listening session" with the FDA and presented our findings, based on scientific, peer reviewed and FDA and CDC documents. The presentation was held on March 12th of 2010 and the silence of the FDA to our findings is deafening.
What I am going to do is inform you as to what our findings are in a series of articles. The reason for this is because the presentation had 54 slides and there is just too much data involved for just one article.
Just a quick note, I have put a definition section at the end of the article because there are items that require further explanation to be understood.
Today, I am going to answer the question "My daughter has received an irregular pap test two years after the vaccination series. I thought the vaccine was supposed to prevent this?" This is something that we are now receiving more and more reports of. Just recently we saw that there is a report on the VAERS data base that stated that a young 32 year old female vaccinated with Cervarix "was diagnosed with adenocarcinoma" (cancer). Here is the write-up from the VAERS (Vaccine Adverse Event Reporting System) report number 385637.
Write-up: This case was reported by a physician via a sales representative and described the occurrence of adenocarcinoma in a 32-year-old female subject who was vaccinated with CERVARIX (GlaxoSmithKline). Relevant medical history includes a post natal check with normal cervix appearance and a pap smear test with normal result. Subsequently, the subject received full course of CERVARIX. On an unspecified date the subject received 1st dose, 2nd dose and 3rd dose of CERVARIX (.5 ml, unknown, lot number not provided). On 3 April 2010, within months of the last vaccination with CERVARIX, the subject experienced post coital bleeding. She was found to have papillary projectary mass on cervix with abnormal appearance. A pap smear and a biopsy were performed and she was diagnosed with adenocarcinoma. The reporting physician considered the events to be clinically significant/intervention required. At the time of reporting the outcome of the events was unspecified.
We are now receiving emails regarding females who have irregular cells detected 2 to 3 years after receiving the vaccination series. Here is example of the emails we are receiving.
By sled****| Reply | (2) replies | Private Message
January 2th, 2009, 8:20 AM
A good friend of mine, a person I have known for 30 years, told me yesterday that her son's girlfriend, a college student, had surgery over the Christmas break for cervical cancer. This is her second surgery, and both surgeries were paid for by the manufacturer of Gardasil. By paying for these surgeries, it seems clear that the company is accepting responsibility for what happened to her. Apparently, because this girl had a weak immune system, Gardasil actually caused the very thing it was intended to prevent — cervical cancer.
Neither my friend nor I had ever heard anything like this before, and I am wondering if others know of girls who have actually gotten cervical cancer after receiving Gardasil.
Presently, According to the National Vaccine Information Center there are 278 events reported to VAERS for HPV, HPV2 and HPV4 when the query is "smear cervix abnormal." http://www.medalerts.org
This is not news to me since the CDC, FDA and ACIP do not recommend testing to see if the HPV vaccination is appropriate. "Females who have abnormalities on their cervical cancer screening results are likely to be infected with one or more genital HPV types. With increasing severity of Papanicolau (Pap) findings, the likelihood of infection with HPV 16 or 18 increases, and benefits of vaccination decrease. Vaccination is still recommended for such females, because vaccination can provide protection against infection with HPV vaccine types not already acquired. Females should be advised that vaccination will have no therapeutic effect on an existing HPV infection or abnormal Pap test."(1)
"Prevaccination assessments (e.g., Pap testing or screening for high-risk HPV DNA, type-specific HPV tests, or HPV antibody) to establish the appropriateness of HPV vaccination are not recommended at any age."(1)
This is what I wrote in regards to these statements.
In the first paragraph that I have from this report section it tells you that if you have HPV 16 or 18 the benefits of the vaccine decrease and the vaccine will have no therapeutic effect. Then they tell you that pre-vaccination assessments (Pap tests) are not recommended at any age. (2)
If you read between the lines they are telling all individuals to get the vaccine that is very expensive and making the manufactures billions of dollars without getting a pap test to see if it will have any benefit to you. In essence this is saying that they do not care if you have HPV at the time of vaccination. Just get the vaccine and make pharma rich. (2)
The research that was carried out in this paper submitted to the FDA was on young women who tested positive for the HPV strains 16 or 18. The facts speak for themselves. The table indicates that the efficacy of Gardasil after vaccinating infected young women dropped to a staggering minus 44.6%. This not only left these women completely vulnerable and without any protection but also has put them, in our opinion, at greater risk of getting HPV CIN2/3 lesions in later life. Using only the Gardasil group this breaks down to 20 out of every 100 women will be diagnosed with CIN 2/3 or worse.
Table 17. Study 013: Applicant's analysis of efficacy against vaccine-relevant HPV types CIN 2/3 or worse among subjects who were PCR positive and seropositive for relevant HPV types at day 1. [From original BLA, study 013 CSR, Table 11-88, p. 636] (3)
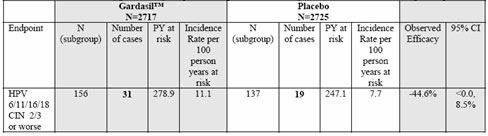
Table 19. Study 013: Analysis of efficacy against vaccine-relevant HPV types CIN 2/3 or worse among subjects who were PCR positive and/or seropositive for the relevant HPV type at day 1. [From additional efficacy analyses requested by CBER and submitted March 15, 2006, table 1e-2, p. 13.] (3)
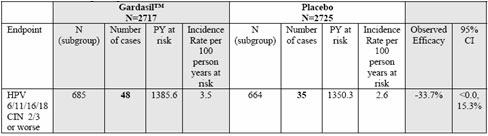
As you can see, the participants that were both PCR positive and seropositive for the HPV types in the vaccine have a 33.7% greater chance of cervical cancer.
The risk of introducing a vaccine concurrently with an infection is unfortunately the trigger to more serious problems in the future.
There is no reason to believe that this will not also apply to Cervarix. The small print on GlaxoSmithKline Cervarix product insert states: "Cervarix does not prevent HPV-related lesions in women infected with HPV 16 or HPV 18 at the time of vaccination and has not been shown to have a therapeutic effect." This statement cannot be much clearer.
Table 15 Study HPV-008: overview of vaccine efficacy against histological lesions associated with HPV-16/18 (by PCR) in HPV DNA positive subjects at baseline (TVC-1)
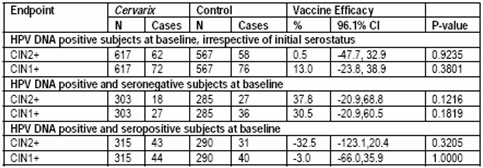
As expected, the vaccine did not show a therapeutic effect in subjects HPV DNA positive at baseline for the type considered in the evaluation (Table 15). Pg 62 (5)
The above data shows that Cervarix has a negative efficacy similar to Gardasil. If a participant was DNA positive and seropositive they are 32.5% more likely to acquire cervical lesions in our opinion.
They even state that the vaccine did not show a therapeutic effect in subjects HPV DNA positive.
Our conclusions where that evidence detailed here regarding the poor efficacy of both Gardasil and Cervarix on already infected women has to be investigated further. If this is occurring in established infected groups of women then what will be occurring in the bodies of adolescent girls who in many cases may already be sexually active and/or be infected at the time of vaccination? In the United States and United Kingdom, HPV SCREENING DOES NOT TAKE PLACE TO DETERMINE IF HPV INFECTION IS ALREADY PRESENT.
"In June 2009, representatives from the Society, ACOG (American College of Obstetricians and Gynecologists), and approximately 25 other organizations met to discuss cervical screening and management for adolescents. There was general consensus that new screening guidelines should recommend against adolescent screening and that screening should begin at age 21," said Saslow. "The Society will formally review the evidence and update our cervical cancer screening recommendations in the coming year." (6)
Pap screening does not start in the United States until the age of 21; Scotland at the age of 20 and in England/Wales at the age of 25. The American College of Obstetricians and Gynecologists "ACOG's new guidelines, state that women 30 and older should be screened for cervical cancer whether by conventional or liquid PAP test once every 2 years, instead of annually as was previously recommended." (6)
It has recently been reported in the UK that teenagers as young as 14 are sexually active; are becoming pregnant and that medical people are encouraging 16 year olds and older to be vaccinated even though they may be sexually active and HPV status will not be tested.
Great risks are being taken with the future of our young people. The promise has been made by Merck and GlaxoSmithKline that in most cases those who are vaccinated will be protected from the HPV strains which cause cervical cancer. The figures presented now state quite clearly that this is not the case.
What bothers me greatly is the fact that the CDC, FDA and ACIP do not recommend that testing should be done to prove whether the HPV vaccination series is appropriate. This lack of concern for the finances of the families and health of our young people has me shaking my head is disgust. Many of the families with young girls or young women suffering from an adverse reaction to the vaccination are spending thousands and thousands of dollars for treatments just to be told it is all in their child's head.
So what we have here is a major problem this is why:
1. An infant can contract HPV at birth
2. A child can contract HPV by lateral transmission
3. Young girls and women are having intercourse at an earlier age
4. New pap recommendations will push initial screenings back to age 25
The information presented thus far indicates that the majority of young girls and women will fall under the category of negative efficacy or no efficacy with regard to the HPV vaccines.
Previously we reported that the figures come to 20 out of every 100 could end up being diagnosed with CIN 2/3 of the cervix. This does not add up considering what the average is per the National Cancer Institute. (7)
From 2003-2007, the median age at diagnosis for cancer of the cervix uteri was 48 years of age2. Approximately 0.2% were diagnosed under age 20; 14.5% between 20 and 34; 26.1% between 35 and 44; 23.7% between 45 and 54; 16.3% between 55 and 64; 10.4% between 65 and 74; 6.5% between 75 and 84; and 2.4% 85+ years of age.
The age-adjusted incidence rate was 8.1 per 100,000 women per year. These rates are based on cases diagnosed in 2003-2007 from 17 SEER (Surveillance Epidemiology and End Results) geographic areas.
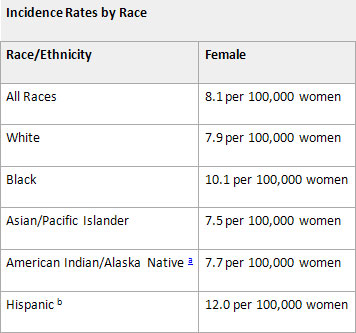
We are looking at the comparison between 20 per 100 as calculated using the available numbers per the reports to the FDA for approval of the vaccinated and 8.1 per 100,000 cited in the report above. Considering we are talking about cancer here I think I would go for the 8.1 to 100,000 and get pap screening every two — three years and save the money that would have been spent on the HPV vaccines for a rainy day.
Considering that both manufacturers reported negative efficacy if positive, there is now the very real risk that these females now have the potential for cervical lesions and cancer at earlier ages than previous studies have determined. When initial pap screening takes place for a woman in her early 20's, an 11 year old who may already have HPV anti-bodies present has the chance of HPV infection prior to the availability of screening. If this infection is not caught early it could lead to cervical lesions and possibly cervical cancer at an early age.
Considering that an infant can come into contact with HPV, what is the benefit to a 9 to 20 year old if testing is not available to them to determine if they are HPV positive or not? NONE!
These vaccines should not be given to young children because no testing is going to be done to see if they are positive for HPV antibodies. Because of this fact the families of these young people could be putting their precious children at risk for cervical cancer earlier in life. Not to mention that their children will not be protected from the HPV types in the vaccine so they are spending their money foolishly for no benefit except to the vaccine manufactures.
The other item that has me concerned is the talk about giving this vaccine to infants. This was reported in the "The Sydney Morning Herald," Cervical vaccine trial on babies: report, August 27, 2007–8:09AM in Australia. © 2007 AAP http://www.smh.com.au/news/National/Cervical-vaccine-trial-on-babies-report/2007/08/27/1188066978713.html
Just a little side note here about what causes the infection which is the reason for the testing.
On the way to looking up other things I find the greatest scientific studies with regards to the HPV. I found that the cause is not what you think it is via the marketing strategies of the pharmaceutical companies. This is what really causes cervical cancer that everyone talks about.
Interleukin la and tumor necrosis factor a stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells (8)
"HPV infections occur frequently in sexually active individuals; however, only a minority of infected women actually develop cervical cancer (7). Thus, additional environmental and/or hereditary factors are involved in malignant progression. A number of other sexually transmitted diseases frequently accompany infection with HPV (8, 9) and cause acute or chronic inflammation within the cervical mucosa"
"Amphiregulin is a member of a large family of polypeptide growth factors that bind and activate the EGF receptor (reviewed in ref. 27). Amphiregulin was originally purified from MCF-7 breast carcinoma cells (28), but it has also been detected in normal tissues, including ovary, placenta, colon, and epidermis (18). Our results implicate amphiregulin as an important autocrine factor that mediates growth stimulation of HPV-immortalized cervical cells by IL-la or TNF-a. This conclusion is based on several observations: (i) both IL-la and TNF-a induced amphiregulin RNA expression in cultured cervical cells; (ii) recombinant amphiregulin stimulated growth of these cells as effectively as IL-la or TNF-a; (iii) a monoclonal antibody that blocks EGF receptor signal transduction completely prevented growth stimulation by IL-la or TNF-a; and (iv) a mixture of monoclonal antibodies that neutralize amphiregulin activity inhibited IL-la- or TNF-amediated proliferation by 95% or 85%, respectively. Autocrine amphiregulin expression is important in supporting autonomous growth of cultured epidermal keratinocytes (18) as well as colon and breast carcinoma cell lines (29, 30). Furthermore, amphiregulin is often overexpressed in malignant colon or mammary tissue relative to the normal epithelia (31, 32), suggesting that altered regulation of this growth factor may contribute to malignant development. Our results demonstrate that two proinflammatory cytokines, IL-la and TNF-a, stimulate proliferation of cervical cells via autocrine induction of amphiregulin.
"These results demonstrate that transfection and immortalization of cervical epithelial cells with HPV-16 or -18 DNA induces sensitivity to growth stimulation by IL-la and TNF-a." However, growth stimulation by these cytokines is not limited to cells containing HPV DNA. Previous studies have shown that several proinflammatory cytokines (IL-la, IL-6, or TNF-a) stimulate proliferation of carcinoma cell lines derived from several different tissues (24, 33, 34) including cervix (35). Thus, proinflammatory cytokines might act as paracrine or autocrine growth factors in promoting malignant progression."
I know that this is for the scientist to understand but this is the stuff that I read every day to find the answers for you. So let me give you the cliff notes of what they are talking about in the above paragraphs.
In the first paragraph they are telling you that "only a minority of infected women actually develop cervical cancer." They attribute this to the real possibility that there are environmental and/or hereditary factors that are coming into play here. If you look at the environmental you have genetically modified foods and vaccines, drinking water with fluoride and chloride, and other chemicals that are too numerous to mention and our air is mildly polluted.
They attribute the infection to STD's other than the HPV types in the vaccines. When you have a persistent infection odds are that those cells are growing because of IL-1a or TNF-a. In turn this activates Amphiregulin which is a "member of a large family of polypeptide growth factors." If you have the activation of Amphiregulin this "mediates growth stimulation of HPV-immortalized cervical cells..."
The other interesting item is that this process is also found in other cancers as well such as the colon and breast cancer. What is distressing to me with this study is that it was written in 1995. This is way before the HPV vaccines. When I was reading this I was thinking about all the people that have died from colon or breast cancer since 1995 and I wanted to cry.
My question here is why are they not addressing the effects of Amphiregulin in regards to cervical and breast cancer? Could it be because in the big scheme of things it is not profitable?
In part 4 of this series I will be answering the question "Why is my daughter so sick now when she was always so healthy before the vaccination?" This question is asked of us so many times that we have lost count a long time ago. In the webinar with the FDA we examined the reports presented to the FDA by Merck and GlaxoSmithKline. We also performed an in-depth analysis of the VAERS reports with some amazing but distressing findings.
If after reading this you want to do your own research into the HPV vaccines here are some places to start.
http://www.renewamerica.com/columns/janak
http://truthaboutgardasil.org
http://www.cynthiajanak.com/Gardasil.html
http://holyhormones.com
Definitions:
CIN — Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the potentially premalignant transformation and abnormal growth (dysplasia) of squamous cells on the surface of the cervix. Most cases of CIN remain stable, or are eliminated by the host's immune system without intervention. http://en.wikipedia.org/wiki/Cervical_intraepithelial_neoplasia
Efficacy — Efficacy is the capacity to produce an effect. In pharmacology, efficacy refers to the maximum response achievable from a drug. The widely used 'intention to treat' method of analysing clinical trials provides estimates of 'use' effectiveness which are typically biased compared with 'method' effectiveness. In a healthcare context, efficacy indicates the capacity for beneficial change (or therapeutic effect) of a given intervention (e.g. a medicine, medical device, surgical procedure, or a public health intervention). http://en.wikipedia.org/wiki/Efficacy
IL-1a — Its initial discovery was as a factor that could induce fever, control lymphocytes, increase the number of bone marrow cells and cause degeneration of bone joints. Both IL-1α and IL-1β form an important part of the inflammatory response of the body against infection. The increased body temperature helps the body's immune system to fight infection. http://en.wikipedia.org/wiki/Il-1a
PCR — The polymerase chain reaction (PCR) is a technique in molecular biology to amplify a single or few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence. http://en.wikipedia.org/wiki/Polymerase_chain_reaction
Seropositive — Seroconversion is the development of detectable specific antibodies to microorganisms in the blood serum as a result of infection or immunization. Serology (the testing for antibodies) is used to determine antibody positivity. Prior to seroconversion, the blood test is seronegative for the antibody; after seroconversion, the blood test is seropositive for the antibody. http://en.wikipedia.org/wiki/Seropositive
TNF-a — Tumor necrosis factor-alpha, Tumor necrosis factor promotes the inflammatory response, which, in turn, causes many of the clinical problems associated with autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis and refractory asthma. http://en.wikipedia.org/wiki/TNF-a
(1) Recommendations from the Advisory Committee on Immunization Practices (ACIP) in regards to Gardasil and Cervarix http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5920a4.htm
(2) Recommendations from the Advisory Committee on Immunization Practices (ACIP) in regards to Gardasil and Cervarix, double speak, Renew America, June 6, 2010 http://www.renewamerica.com/columns/janak/100606
(3) Clinical Review of Biologics License Application for Human Papillomavirus, June 8, 2006
(4) Clinical Review of Biologics License Application Supplement for Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18), September 11, 2008
(5) CERVARIX, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, Recombinant Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document, September 9, 2009
(6) ACOG Revises Cervical Cancer Screening Guidelines, Article date: 2009/11/20, http://www.cancer.org/docroot/NWS/content/NWS_1_1x_ACOG_Revises_Cervical_Cancer_Screening_Guidelines.asp
(7) National Cancer Institute — Surveillance Epidemiology and End Results, http://seer.cancer.gov/statfacts/html/cervix.html
(8) Proc. Natl. Acad. Sci. USA Vol. 92, pp. 2840-2844, March 1995 Cell Biology, Interleukin la and tumor necrosis factor a stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells, http://www.pnas.org/content/92/7/2840.full.pdf
© Cynthia A. Janak
June 16, 2010
For those of you who have come here for the first time let me inform you as to why I am writing this series. I was part of an international group of women and we had a "listening session" with the FDA and presented our findings, based on scientific, peer reviewed and FDA and CDC documents. The presentation was held on March 12th of 2010 and the silence of the FDA to our findings is deafening.
What I am going to do is inform you as to what our findings are in a series of articles. The reason for this is because the presentation had 54 slides and there is just too much data involved for just one article.
Just a quick note, I have put a definition section at the end of the article because there are items that require further explanation to be understood.
Today, I am going to answer the question "My daughter has received an irregular pap test two years after the vaccination series. I thought the vaccine was supposed to prevent this?" This is something that we are now receiving more and more reports of. Just recently we saw that there is a report on the VAERS data base that stated that a young 32 year old female vaccinated with Cervarix "was diagnosed with adenocarcinoma" (cancer). Here is the write-up from the VAERS (Vaccine Adverse Event Reporting System) report number 385637.
Write-up: This case was reported by a physician via a sales representative and described the occurrence of adenocarcinoma in a 32-year-old female subject who was vaccinated with CERVARIX (GlaxoSmithKline). Relevant medical history includes a post natal check with normal cervix appearance and a pap smear test with normal result. Subsequently, the subject received full course of CERVARIX. On an unspecified date the subject received 1st dose, 2nd dose and 3rd dose of CERVARIX (.5 ml, unknown, lot number not provided). On 3 April 2010, within months of the last vaccination with CERVARIX, the subject experienced post coital bleeding. She was found to have papillary projectary mass on cervix with abnormal appearance. A pap smear and a biopsy were performed and she was diagnosed with adenocarcinoma. The reporting physician considered the events to be clinically significant/intervention required. At the time of reporting the outcome of the events was unspecified.
We are now receiving emails regarding females who have irregular cells detected 2 to 3 years after receiving the vaccination series. Here is example of the emails we are receiving.
By sled****| Reply | (2) replies | Private Message
January 2th, 2009, 8:20 AM
A good friend of mine, a person I have known for 30 years, told me yesterday that her son's girlfriend, a college student, had surgery over the Christmas break for cervical cancer. This is her second surgery, and both surgeries were paid for by the manufacturer of Gardasil. By paying for these surgeries, it seems clear that the company is accepting responsibility for what happened to her. Apparently, because this girl had a weak immune system, Gardasil actually caused the very thing it was intended to prevent — cervical cancer.
Neither my friend nor I had ever heard anything like this before, and I am wondering if others know of girls who have actually gotten cervical cancer after receiving Gardasil.
Presently, According to the National Vaccine Information Center there are 278 events reported to VAERS for HPV, HPV2 and HPV4 when the query is "smear cervix abnormal." http://www.medalerts.org
This is not news to me since the CDC, FDA and ACIP do not recommend testing to see if the HPV vaccination is appropriate. "Females who have abnormalities on their cervical cancer screening results are likely to be infected with one or more genital HPV types. With increasing severity of Papanicolau (Pap) findings, the likelihood of infection with HPV 16 or 18 increases, and benefits of vaccination decrease. Vaccination is still recommended for such females, because vaccination can provide protection against infection with HPV vaccine types not already acquired. Females should be advised that vaccination will have no therapeutic effect on an existing HPV infection or abnormal Pap test."(1)
"Prevaccination assessments (e.g., Pap testing or screening for high-risk HPV DNA, type-specific HPV tests, or HPV antibody) to establish the appropriateness of HPV vaccination are not recommended at any age."(1)
This is what I wrote in regards to these statements.
In the first paragraph that I have from this report section it tells you that if you have HPV 16 or 18 the benefits of the vaccine decrease and the vaccine will have no therapeutic effect. Then they tell you that pre-vaccination assessments (Pap tests) are not recommended at any age. (2)
If you read between the lines they are telling all individuals to get the vaccine that is very expensive and making the manufactures billions of dollars without getting a pap test to see if it will have any benefit to you. In essence this is saying that they do not care if you have HPV at the time of vaccination. Just get the vaccine and make pharma rich. (2)
The research that was carried out in this paper submitted to the FDA was on young women who tested positive for the HPV strains 16 or 18. The facts speak for themselves. The table indicates that the efficacy of Gardasil after vaccinating infected young women dropped to a staggering minus 44.6%. This not only left these women completely vulnerable and without any protection but also has put them, in our opinion, at greater risk of getting HPV CIN2/3 lesions in later life. Using only the Gardasil group this breaks down to 20 out of every 100 women will be diagnosed with CIN 2/3 or worse.
Table 17. Study 013: Applicant's analysis of efficacy against vaccine-relevant HPV types CIN 2/3 or worse among subjects who were PCR positive and seropositive for relevant HPV types at day 1. [From original BLA, study 013 CSR, Table 11-88, p. 636] (3)

Table 19. Study 013: Analysis of efficacy against vaccine-relevant HPV types CIN 2/3 or worse among subjects who were PCR positive and/or seropositive for the relevant HPV type at day 1. [From additional efficacy analyses requested by CBER and submitted March 15, 2006, table 1e-2, p. 13.] (3)

As you can see, the participants that were both PCR positive and seropositive for the HPV types in the vaccine have a 33.7% greater chance of cervical cancer.
The risk of introducing a vaccine concurrently with an infection is unfortunately the trigger to more serious problems in the future.
There is no reason to believe that this will not also apply to Cervarix. The small print on GlaxoSmithKline Cervarix product insert states: "Cervarix does not prevent HPV-related lesions in women infected with HPV 16 or HPV 18 at the time of vaccination and has not been shown to have a therapeutic effect." This statement cannot be much clearer.
Table 15 Study HPV-008: overview of vaccine efficacy against histological lesions associated with HPV-16/18 (by PCR) in HPV DNA positive subjects at baseline (TVC-1)

As expected, the vaccine did not show a therapeutic effect in subjects HPV DNA positive at baseline for the type considered in the evaluation (Table 15). Pg 62 (5)
The above data shows that Cervarix has a negative efficacy similar to Gardasil. If a participant was DNA positive and seropositive they are 32.5% more likely to acquire cervical lesions in our opinion.
They even state that the vaccine did not show a therapeutic effect in subjects HPV DNA positive.
Our conclusions where that evidence detailed here regarding the poor efficacy of both Gardasil and Cervarix on already infected women has to be investigated further. If this is occurring in established infected groups of women then what will be occurring in the bodies of adolescent girls who in many cases may already be sexually active and/or be infected at the time of vaccination? In the United States and United Kingdom, HPV SCREENING DOES NOT TAKE PLACE TO DETERMINE IF HPV INFECTION IS ALREADY PRESENT.
"In June 2009, representatives from the Society, ACOG (American College of Obstetricians and Gynecologists), and approximately 25 other organizations met to discuss cervical screening and management for adolescents. There was general consensus that new screening guidelines should recommend against adolescent screening and that screening should begin at age 21," said Saslow. "The Society will formally review the evidence and update our cervical cancer screening recommendations in the coming year." (6)
Pap screening does not start in the United States until the age of 21; Scotland at the age of 20 and in England/Wales at the age of 25. The American College of Obstetricians and Gynecologists "ACOG's new guidelines, state that women 30 and older should be screened for cervical cancer whether by conventional or liquid PAP test once every 2 years, instead of annually as was previously recommended." (6)
It has recently been reported in the UK that teenagers as young as 14 are sexually active; are becoming pregnant and that medical people are encouraging 16 year olds and older to be vaccinated even though they may be sexually active and HPV status will not be tested.
Great risks are being taken with the future of our young people. The promise has been made by Merck and GlaxoSmithKline that in most cases those who are vaccinated will be protected from the HPV strains which cause cervical cancer. The figures presented now state quite clearly that this is not the case.
What bothers me greatly is the fact that the CDC, FDA and ACIP do not recommend that testing should be done to prove whether the HPV vaccination series is appropriate. This lack of concern for the finances of the families and health of our young people has me shaking my head is disgust. Many of the families with young girls or young women suffering from an adverse reaction to the vaccination are spending thousands and thousands of dollars for treatments just to be told it is all in their child's head.
So what we have here is a major problem this is why:
1. An infant can contract HPV at birth
2. A child can contract HPV by lateral transmission
3. Young girls and women are having intercourse at an earlier age
4. New pap recommendations will push initial screenings back to age 25
The information presented thus far indicates that the majority of young girls and women will fall under the category of negative efficacy or no efficacy with regard to the HPV vaccines.
Previously we reported that the figures come to 20 out of every 100 could end up being diagnosed with CIN 2/3 of the cervix. This does not add up considering what the average is per the National Cancer Institute. (7)
From 2003-2007, the median age at diagnosis for cancer of the cervix uteri was 48 years of age2. Approximately 0.2% were diagnosed under age 20; 14.5% between 20 and 34; 26.1% between 35 and 44; 23.7% between 45 and 54; 16.3% between 55 and 64; 10.4% between 65 and 74; 6.5% between 75 and 84; and 2.4% 85+ years of age.
The age-adjusted incidence rate was 8.1 per 100,000 women per year. These rates are based on cases diagnosed in 2003-2007 from 17 SEER (Surveillance Epidemiology and End Results) geographic areas.

We are looking at the comparison between 20 per 100 as calculated using the available numbers per the reports to the FDA for approval of the vaccinated and 8.1 per 100,000 cited in the report above. Considering we are talking about cancer here I think I would go for the 8.1 to 100,000 and get pap screening every two — three years and save the money that would have been spent on the HPV vaccines for a rainy day.
Considering that both manufacturers reported negative efficacy if positive, there is now the very real risk that these females now have the potential for cervical lesions and cancer at earlier ages than previous studies have determined. When initial pap screening takes place for a woman in her early 20's, an 11 year old who may already have HPV anti-bodies present has the chance of HPV infection prior to the availability of screening. If this infection is not caught early it could lead to cervical lesions and possibly cervical cancer at an early age.
Considering that an infant can come into contact with HPV, what is the benefit to a 9 to 20 year old if testing is not available to them to determine if they are HPV positive or not? NONE!
These vaccines should not be given to young children because no testing is going to be done to see if they are positive for HPV antibodies. Because of this fact the families of these young people could be putting their precious children at risk for cervical cancer earlier in life. Not to mention that their children will not be protected from the HPV types in the vaccine so they are spending their money foolishly for no benefit except to the vaccine manufactures.
The other item that has me concerned is the talk about giving this vaccine to infants. This was reported in the "The Sydney Morning Herald," Cervical vaccine trial on babies: report, August 27, 2007–8:09AM in Australia. © 2007 AAP http://www.smh.com.au/news/National/Cervical-vaccine-trial-on-babies-report/2007/08/27/1188066978713.html
Just a little side note here about what causes the infection which is the reason for the testing.
On the way to looking up other things I find the greatest scientific studies with regards to the HPV. I found that the cause is not what you think it is via the marketing strategies of the pharmaceutical companies. This is what really causes cervical cancer that everyone talks about.
Interleukin la and tumor necrosis factor a stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells (8)
"HPV infections occur frequently in sexually active individuals; however, only a minority of infected women actually develop cervical cancer (7). Thus, additional environmental and/or hereditary factors are involved in malignant progression. A number of other sexually transmitted diseases frequently accompany infection with HPV (8, 9) and cause acute or chronic inflammation within the cervical mucosa"
"Amphiregulin is a member of a large family of polypeptide growth factors that bind and activate the EGF receptor (reviewed in ref. 27). Amphiregulin was originally purified from MCF-7 breast carcinoma cells (28), but it has also been detected in normal tissues, including ovary, placenta, colon, and epidermis (18). Our results implicate amphiregulin as an important autocrine factor that mediates growth stimulation of HPV-immortalized cervical cells by IL-la or TNF-a. This conclusion is based on several observations: (i) both IL-la and TNF-a induced amphiregulin RNA expression in cultured cervical cells; (ii) recombinant amphiregulin stimulated growth of these cells as effectively as IL-la or TNF-a; (iii) a monoclonal antibody that blocks EGF receptor signal transduction completely prevented growth stimulation by IL-la or TNF-a; and (iv) a mixture of monoclonal antibodies that neutralize amphiregulin activity inhibited IL-la- or TNF-amediated proliferation by 95% or 85%, respectively. Autocrine amphiregulin expression is important in supporting autonomous growth of cultured epidermal keratinocytes (18) as well as colon and breast carcinoma cell lines (29, 30). Furthermore, amphiregulin is often overexpressed in malignant colon or mammary tissue relative to the normal epithelia (31, 32), suggesting that altered regulation of this growth factor may contribute to malignant development. Our results demonstrate that two proinflammatory cytokines, IL-la and TNF-a, stimulate proliferation of cervical cells via autocrine induction of amphiregulin.
"These results demonstrate that transfection and immortalization of cervical epithelial cells with HPV-16 or -18 DNA induces sensitivity to growth stimulation by IL-la and TNF-a." However, growth stimulation by these cytokines is not limited to cells containing HPV DNA. Previous studies have shown that several proinflammatory cytokines (IL-la, IL-6, or TNF-a) stimulate proliferation of carcinoma cell lines derived from several different tissues (24, 33, 34) including cervix (35). Thus, proinflammatory cytokines might act as paracrine or autocrine growth factors in promoting malignant progression."
I know that this is for the scientist to understand but this is the stuff that I read every day to find the answers for you. So let me give you the cliff notes of what they are talking about in the above paragraphs.
In the first paragraph they are telling you that "only a minority of infected women actually develop cervical cancer." They attribute this to the real possibility that there are environmental and/or hereditary factors that are coming into play here. If you look at the environmental you have genetically modified foods and vaccines, drinking water with fluoride and chloride, and other chemicals that are too numerous to mention and our air is mildly polluted.
They attribute the infection to STD's other than the HPV types in the vaccines. When you have a persistent infection odds are that those cells are growing because of IL-1a or TNF-a. In turn this activates Amphiregulin which is a "member of a large family of polypeptide growth factors." If you have the activation of Amphiregulin this "mediates growth stimulation of HPV-immortalized cervical cells..."
The other interesting item is that this process is also found in other cancers as well such as the colon and breast cancer. What is distressing to me with this study is that it was written in 1995. This is way before the HPV vaccines. When I was reading this I was thinking about all the people that have died from colon or breast cancer since 1995 and I wanted to cry.
My question here is why are they not addressing the effects of Amphiregulin in regards to cervical and breast cancer? Could it be because in the big scheme of things it is not profitable?
In part 4 of this series I will be answering the question "Why is my daughter so sick now when she was always so healthy before the vaccination?" This question is asked of us so many times that we have lost count a long time ago. In the webinar with the FDA we examined the reports presented to the FDA by Merck and GlaxoSmithKline. We also performed an in-depth analysis of the VAERS reports with some amazing but distressing findings.
If after reading this you want to do your own research into the HPV vaccines here are some places to start.
http://www.renewamerica.com/columns/janak
http://truthaboutgardasil.org
http://www.cynthiajanak.com/Gardasil.html
http://holyhormones.com
Definitions:
CIN — Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the potentially premalignant transformation and abnormal growth (dysplasia) of squamous cells on the surface of the cervix. Most cases of CIN remain stable, or are eliminated by the host's immune system without intervention. http://en.wikipedia.org/wiki/Cervical_intraepithelial_neoplasia
Efficacy — Efficacy is the capacity to produce an effect. In pharmacology, efficacy refers to the maximum response achievable from a drug. The widely used 'intention to treat' method of analysing clinical trials provides estimates of 'use' effectiveness which are typically biased compared with 'method' effectiveness. In a healthcare context, efficacy indicates the capacity for beneficial change (or therapeutic effect) of a given intervention (e.g. a medicine, medical device, surgical procedure, or a public health intervention). http://en.wikipedia.org/wiki/Efficacy
IL-1a — Its initial discovery was as a factor that could induce fever, control lymphocytes, increase the number of bone marrow cells and cause degeneration of bone joints. Both IL-1α and IL-1β form an important part of the inflammatory response of the body against infection. The increased body temperature helps the body's immune system to fight infection. http://en.wikipedia.org/wiki/Il-1a
PCR — The polymerase chain reaction (PCR) is a technique in molecular biology to amplify a single or few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence. http://en.wikipedia.org/wiki/Polymerase_chain_reaction
Seropositive — Seroconversion is the development of detectable specific antibodies to microorganisms in the blood serum as a result of infection or immunization. Serology (the testing for antibodies) is used to determine antibody positivity. Prior to seroconversion, the blood test is seronegative for the antibody; after seroconversion, the blood test is seropositive for the antibody. http://en.wikipedia.org/wiki/Seropositive
TNF-a — Tumor necrosis factor-alpha, Tumor necrosis factor promotes the inflammatory response, which, in turn, causes many of the clinical problems associated with autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis and refractory asthma. http://en.wikipedia.org/wiki/TNF-a
(1) Recommendations from the Advisory Committee on Immunization Practices (ACIP) in regards to Gardasil and Cervarix http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5920a4.htm
(2) Recommendations from the Advisory Committee on Immunization Practices (ACIP) in regards to Gardasil and Cervarix, double speak, Renew America, June 6, 2010 http://www.renewamerica.com/columns/janak/100606
(3) Clinical Review of Biologics License Application for Human Papillomavirus, June 8, 2006
(4) Clinical Review of Biologics License Application Supplement for Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18), September 11, 2008
(5) CERVARIX, Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, Recombinant Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document, September 9, 2009
(6) ACOG Revises Cervical Cancer Screening Guidelines, Article date: 2009/11/20, http://www.cancer.org/docroot/NWS/content/NWS_1_1x_ACOG_Revises_Cervical_Cancer_Screening_Guidelines.asp
(7) National Cancer Institute — Surveillance Epidemiology and End Results, http://seer.cancer.gov/statfacts/html/cervix.html
(8) Proc. Natl. Acad. Sci. USA Vol. 92, pp. 2840-2844, March 1995 Cell Biology, Interleukin la and tumor necrosis factor a stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells, http://www.pnas.org/content/92/7/2840.full.pdf
© Cynthia A. Janak
The views expressed by RenewAmerica columnists are their own and do not necessarily reflect the position of RenewAmerica or its affiliates.
(See RenewAmerica's publishing standards.)


















